Dreaming of a safe pet food? No choice: you must know the future!
In the cruel world of pet food safety management, THE rule for limiting risk is to imagine all the hazards creeping around your product and do everything possible to avoid them.
But unfortunately, in real life, whatever energy you expend or tons of disinfectant you put on your equipment, you can’t avoid all the dangers. And it can happen that your product meets nasty bacteria, molds, or yeasts.
How would my product react if confronted with Salmonella? Which antimicrobial solution should I use to save it?
Challenge studies can answer these questions, better than a fortune teller.
Discover how…
What is microbial contamination?
Microbial contamination is the presence of unwanted bacteria, mold, or yeast, in a product. In pet food, if the conditions are favorable, some harmful microorganisms can develop and cause digestive issues or even foodborne illness, in pets… and their parents. This in turn can create negative effects to the pet food companies.
Pet food infection occurs through a few common routes such as contaminated incoming raw materials, contaminated equipment, and poor sanitization practices. To protect the product and limit contamination risks, antimicrobial solutions are usually added at various steps of manufacturing process.
What is a challenge study and why run one?
A challenge study is a testing method in which a product is intentionally inoculated with a contaminant and kept for a specific period. During storage, small samples of the product are tested on various days to determine how the organism develops overtime.
This predictive method is mainly used to show effectiveness of adding an antimicrobial solution in a product, or to compare the effectiveness of various antimicrobial solutions.
Challenge studies can be used for finished pet food and treats but also in fats, meals, or other risky ingredients included in a pet food formula.
How does a challenge study work?
A challenge test generally consists of following phases:
- Inoculation of the contaminant
- Adaptation of the microorganism to its environment
- Baseline count of the contaminant population
- Addition of antimicrobial solution
- Storage with contaminant population counts made at predetermined time points
Detailed test conditions, such as contaminant and antimicrobial agent dosing, as well as storage and analysis conditions, must be carefully determined prior to the study.
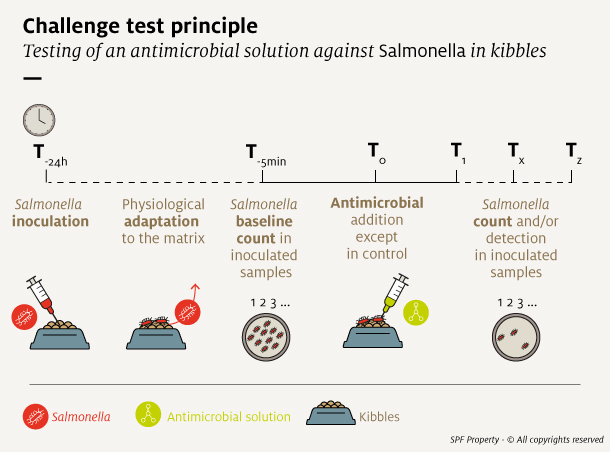
What are the key points for setting up a challenge study?
1- Targeting the enemy
The first step when designing a challenge study is to select the organism that will be inoculated.
The most common contaminants found in pet food include bacteria such as Salmonella, E. coli, Listeria, Clostridium, and fungi such as yeasts and molds. Their presence and development mainly depend on the product type and composition.
For instance, Salmonella is more of a concern within poultry whereas Listeria and Clostridium are more of a concern with bovine. Salmonella is also the most frequent pathogen in kibbles and poultry fat, while raw meat products worry about Listeria, and canned products have a Clostridium risk.
The idea, of course, is to choose the species most likely to contaminate the product under study, based on risk assessment and experience.
2- Choosing the dosages
The level of contaminant used in a challenge study is typically higher than what would be observed in a real-life environment. The hypothesis is that if the antimicrobial is effective at a worst-case value, it will also be effective at a more “normal” value.
On the other hand, the dosages of the antimicrobial agent(s) tested are generally close to those that should be used in real conditions. The idea is to find the best balance between protection effectiveness, cost, and application constraints.
NB: during the storage time, it’s possible to re-inoculate the product with the selected pathogen. It enables to assess the protective effect of the tested antimicrobial solution against recontamination.
3- Selecting length, setting frequency
The length of storage and the frequency of analysis depends on the organism challenged and the product type.
For instance, bacteria can grow relatively fast, whereas molds take time to grow. It might be relevant to have more frequent counts for bacteria to have a more precise view of microbe development kinetic.
In all cases, the study can be ended as soon as the tested product value overcome authorized values, or once the desired shelf-life has been reached.
NB: Challenge studies are in vitro tests, designed to assess the effectiveness of antimicrobial solutions to protect pet food product. Real-time shelf-life studies should be completed with finished pet food to show no long-lasting affect and no negative impact from selected antimicrobial solutions on product or animal health.
What do the results of a challenge study look like?
To estimate microbial population size, samples of the inoculated product taken over time are transferred and grown on agar plates. The monitored indicator is the quantity of Colony-Forming Units (CFU) which estimates the number of viable cells, ie the number of cells capable of growing and multiplicate. This value is generally expressed in log10 for easy reading of large numbers.
The population size counted at each time point is plotted into a growth curve and analyzed to determine organism growth rate. It allows to determine if the addition of an antimicrobial, and at which dosage level, was effective.
Several curve profiles can be observed according to the microorganism and the antimicrobial solution used. The antimicrobial solution can slow the growth of the contaminant – inhibitory effect – or destroy it – killing effect.
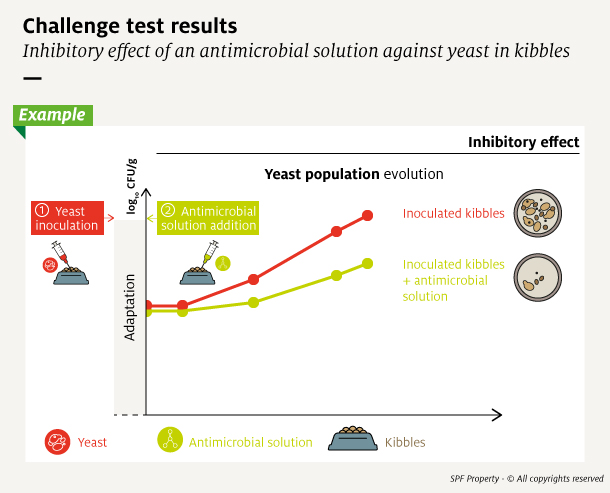
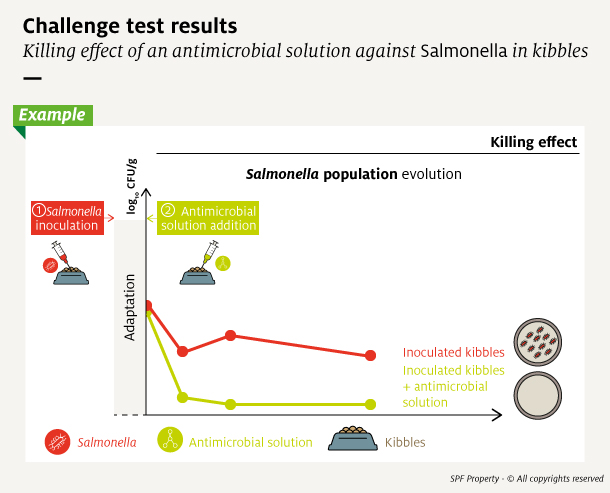
Whatever their mode of action, antimicrobial solutions are considered effective if they ensure that the microbial population does not exceed authorized values, or the antimicrobial solution inhibits growth beyond the desired shelf-life.
Where to run a challenge study?
Pet food manufacturers have the choice to conduct challenge studies in proprietary facilities, in dedicated external laboratories, or in antimicrobial suppliers’ lab.
Wherever the laboratory, it must be able to manage live microbial cultures and meet definite biosafety levels criteria.
Moreover, given the high safety and image risks associated with microbial contamination of pet food, the results of a challenge study must be reliable.
For pet food manufacturers, it could be safer and easier to work with outside facilities with specific expertise.
Take-home points





* required fields