Just like you, food products cannot escape the effect of time. Cheese and wine improve with age; however, this is unfortunately not the case for pet food.
Between the time a kibble comes out of the extruder and the time it lands in Kitty’s plate, several months can elapse. During this journey, the inevitable oxidation attack can affect nutritional properties and turn nice poultry scent to rancid smell, which is far less pleasing.
Pet food manufacturers thus need to be aware of and master the oxidative stability of their product to ensure it stays nutritious and appealing to Kitty, and her Human.
What are the methods to assess pet food oxidative stability? What are the oxidation markers to track?
Follow the steps to fully measure pet food oxidation over time…
Oxidative stability: choose the right indicators
Fat oxidation is a common phenomenon affecting the quality of aging pet food. Mainly caused by high temperature or contact with oxygen, this complex set of reactions creates oxidation compounds responsible for rancid smells, off-flavors, and loss of nutritional value.
Antioxidants are commonly added to pet food to limit this phenomenon. Their level decreases over time as they are consumed by reactions with oxidation compounds.
The levels of specific oxidation compounds and residual antioxidants in an aging pet food can relevantly be used as indicators to assess oxidative stability over time.
Chasing oxidation compounds
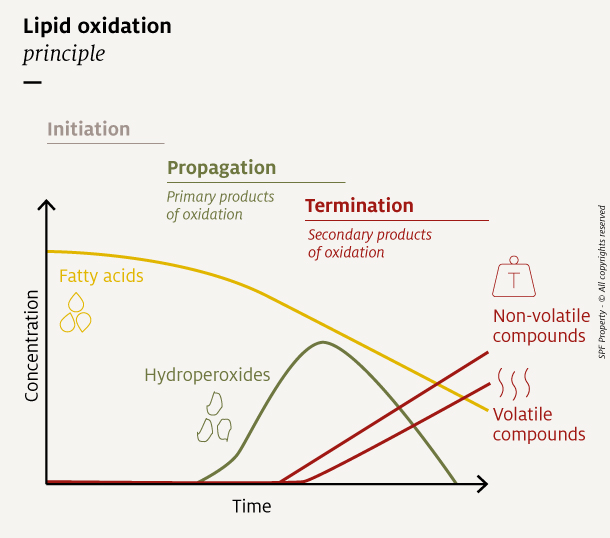
The fat oxidation mechanism happening in pet food can be summarized in 3 stages: initiation, propagation, and termination. Each reaction stage generates different oxidation compounds.
During the initiation and propagation phases, fatty acids turn into free radicals and hydroperoxides, both of which are primary compounds of oxidation.
During the termination phase, hydroperoxides decompose into secondary products of oxidation. They include volatile compounds like aldehydes, ketones, and short chain acids, which are responsible for unpleasant odors, as well as non-volatile compounds that mainly impact taste.
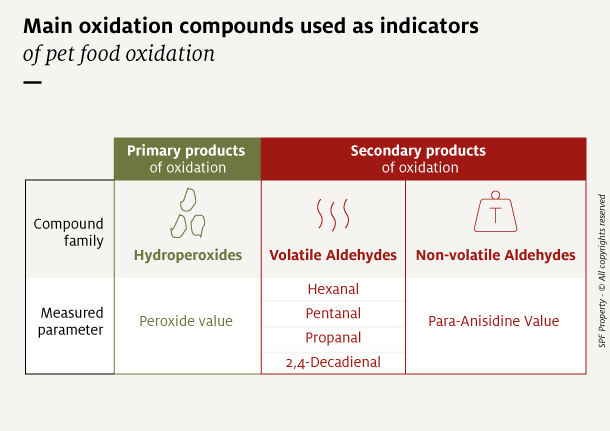
Many compounds generated during oxidation are currently used as indicators of oxidation state. Of these, 3 categories, both primary and secondary, are best suited to complex matrices such as pet foods:
- The hydroperoxides measured with the Peroxide Value, expressed as milliequivalents oxygen per kg of fat (meq/kg fat).
- Volatile aldehydes like hexanal, pentanal, propanal and 2,4-decadienal, measured with Gas chromatography and expressed in ppm.
- Non-volatile aldehydes, measured with the para-Anisidine Value (no unit).
Each indicator provides different and complementary information. To get a complete picture of a product’s oxidation state, it is best to track several oxidation compounds during a kinetic study.
Measuring residual antioxidants level
Antioxidants protect food by delaying oxidative reactions. According to their nature they act differently. They can trap metals and oxygen in the initiation phase, or catch free radicals during the propagation step, thus limiting the generation of unwanted molecules.
When several antioxidants are used, they are consumed at various rates, until there are none left, or the food is consumed. Antioxidant residual level assessed against initial level is a good indicator of the oxidation status of the pet food.
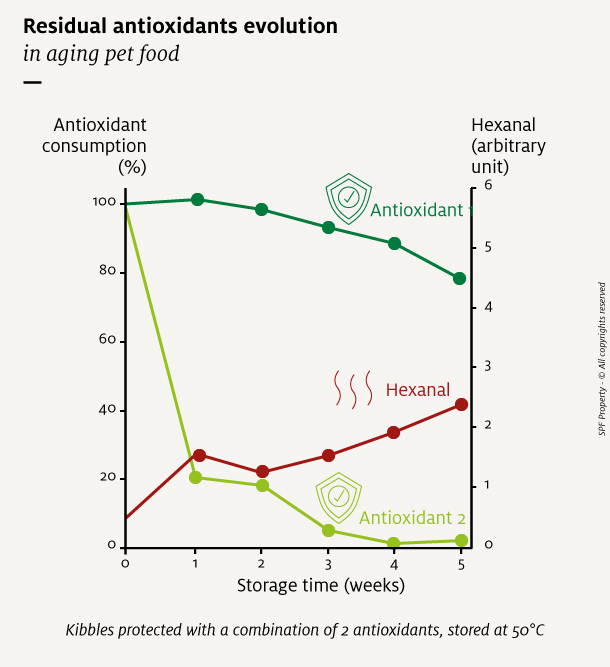
All the antioxidants used in a product should be monitored, as they act at different stages of the oxidation reaction.
Oxidative stability study: choose the method
Once relevant oxidation indicators have been selected, it is possible to set a protocol to follow the oxidation evolution. Two main methods exist to assess the oxidative stability of a food product over time. The major difference is based on the length of the study.
- Real-time study: the product is stored in ambient condition, for as long as its expected shelf life. Its oxidative status is tested throughout the storage duration, with at least 6 time points.
- Accelerated study: the product is stored in an incubator, at a higher temperature (40°C to 60°C), and sometimes with a higher relative humidity. This method speeds up reactions and therefore reduces the duration of the study by 4 to 5 times
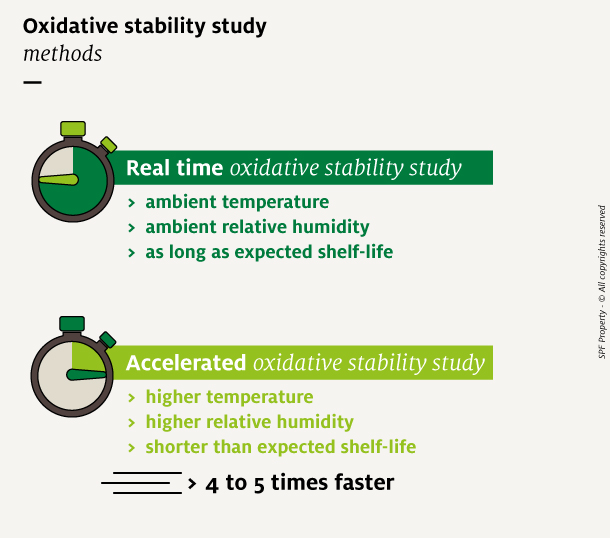
The choice of the protocol is often made according to time available, cost constraints and product strategic importance.
The real time study is the most accurate, but also the most time-consuming, thus more expensive. If a choice needs to be made, it could be reserved for the launch of a new product or a major change in formula.
The accelerated study is a good option which shortens the testing time from several months to several weeks, while still being reliable, especially to compare a current antioxidant to a new one.
It is a best practice to simultaneously run both a real-time and an accelerated study, so that if a problem occurs in the accelerated study, the real-time study can be stopped earlier.
Whether for a real time or an accelerated study, the testing conditions such as the storage duration, the temperature, the indicators, and the frequency of analysis must be carefully chosen to be the most representative of the product’s future life.
Take-home points





* required fields